
Important Questions class 11 Chemistry Some Basic Concepts of Chemistry
Topics and Subtopics in NCERT Solutions for Class 11 Chemistry Chapter 12 Organic Chemistry Some Basic Principles and Techniques: Section Name. Topic Name. 12. Organic Chemistry - Some Basic Principles and Techniques. 12.1. General Introduction. 12.2. Tetravalence of Carbon: Shapes of Organic Compounds.
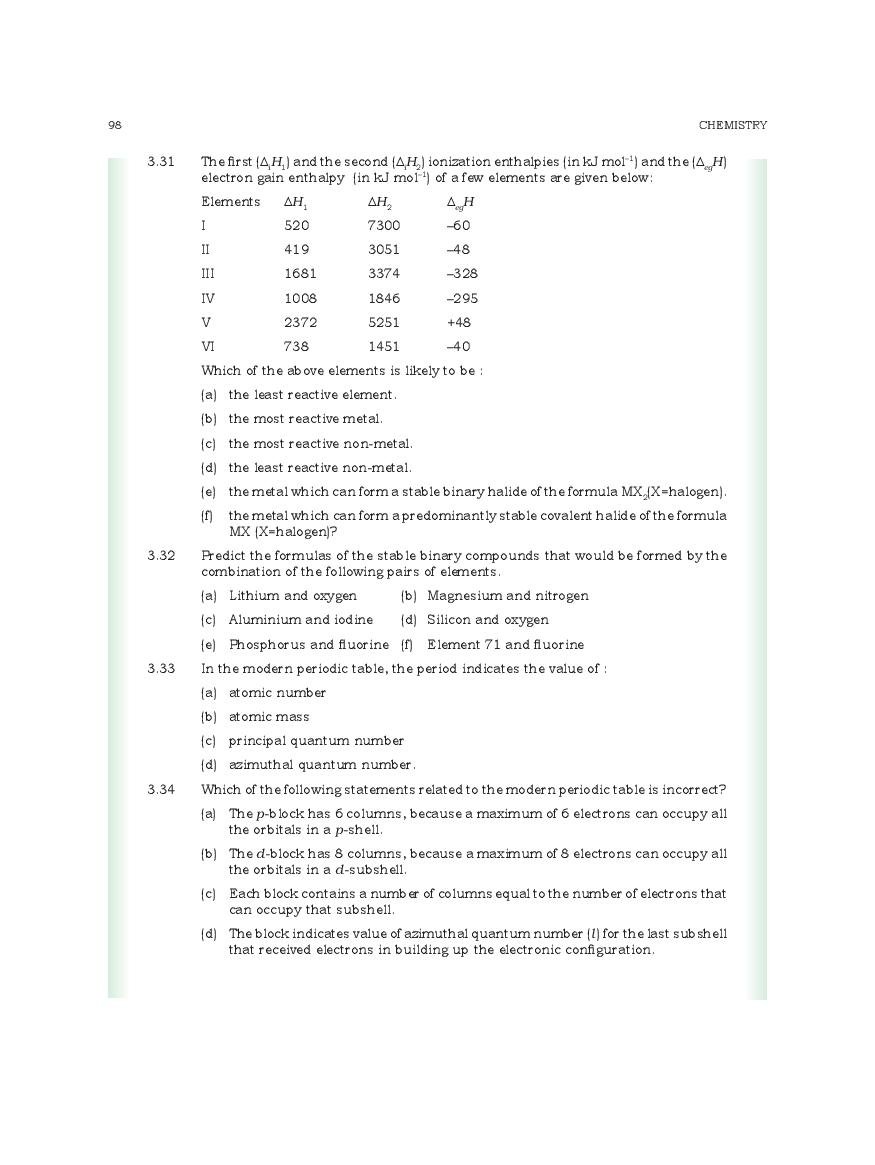
CBSE Class 11 Chemistry Chapter 3 Classification Of Elements And
Question 1. An element is present in the third period of the p-block. It has 5 electrons in its outermost shell. Predict its group. How many unpaired electrons does it have? Answer: It belongs to the 15th group (P). It has 3 unpaired electrons. Question 2. An element X with Z = 112 has been recently discovered.

NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of
Chemistry Important Questions Class 11 are given below chapter wise. Some Basic Concepts of Chemistry. Structure of Atom. Classification of Elements and Periodicity in Properties. Chemical Bonding and Molecular Structure. States of Matter. Thermodynamics.
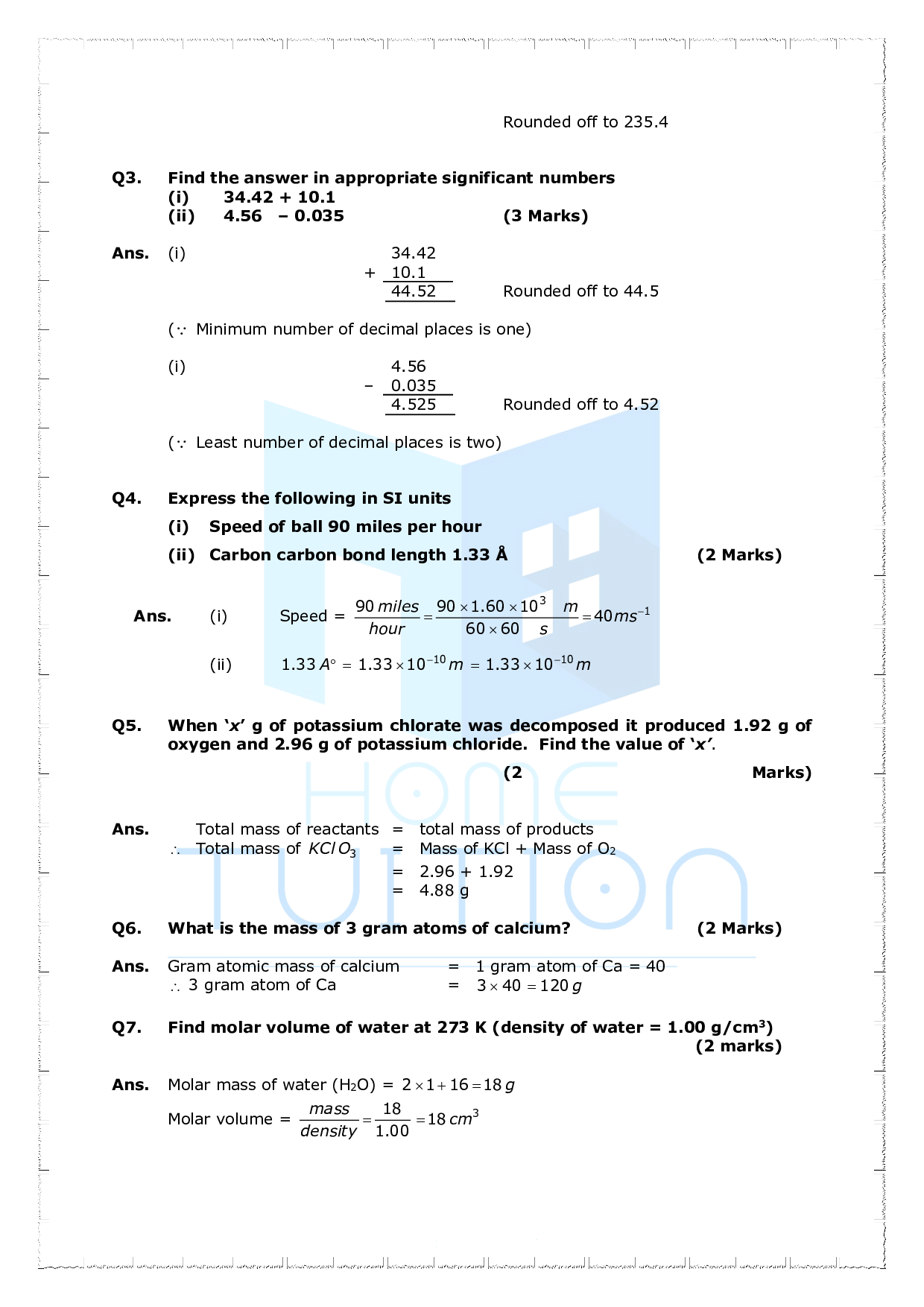
Important Questions For Class 11 Chemistry Chapter 1 Some Basic
on August 19, 2023, 11:05 AM. NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Hindi and English Medium modified and revised for academic year 2023-24. Class 11 Chemistry chapter 3 MCQ, exercise and intext question answers in Properties are updated for new CBSE session 2023-24.

Class 11 Chemistry Worksheet on Chapter 1 Some Basic Concepts of
1. Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine. Answer. This is due to the small size of the fluorine atom. As a result of the strong interelectronic repulsions in fluorine's relatively small 2p orbitals, the incoming electron does not experience much attraction. 2.

Ncert Solutions For Class 11 Chemistry Chapter 3 Classification Of Riset
NCERT Solutions NCERT Class 11 NCERT 11 Chemistry Chapter 3: Classification Of Elements And Periodicity NCERT Solutions for Class 11 Chemistry Chapter 3: Classification of Elements and Periodicity in Properties NCERT Solutions Class 11 Chemistry Chapter 3 - Free PDF Download
Class 11 chemistry chapter 3 solutions CBSE Solutions
1. Explain the non linear shape of H 2 S and non planar shape of PCl 3 using valence shell electron pair repulsion theory. Answer. The main atom in H 2 S is S, which has two lone pairs. These lone pairs cause repulsion and displace the H-S bond, resulting in a non-linear shape. PCl 3 has a trigonal planar structure.
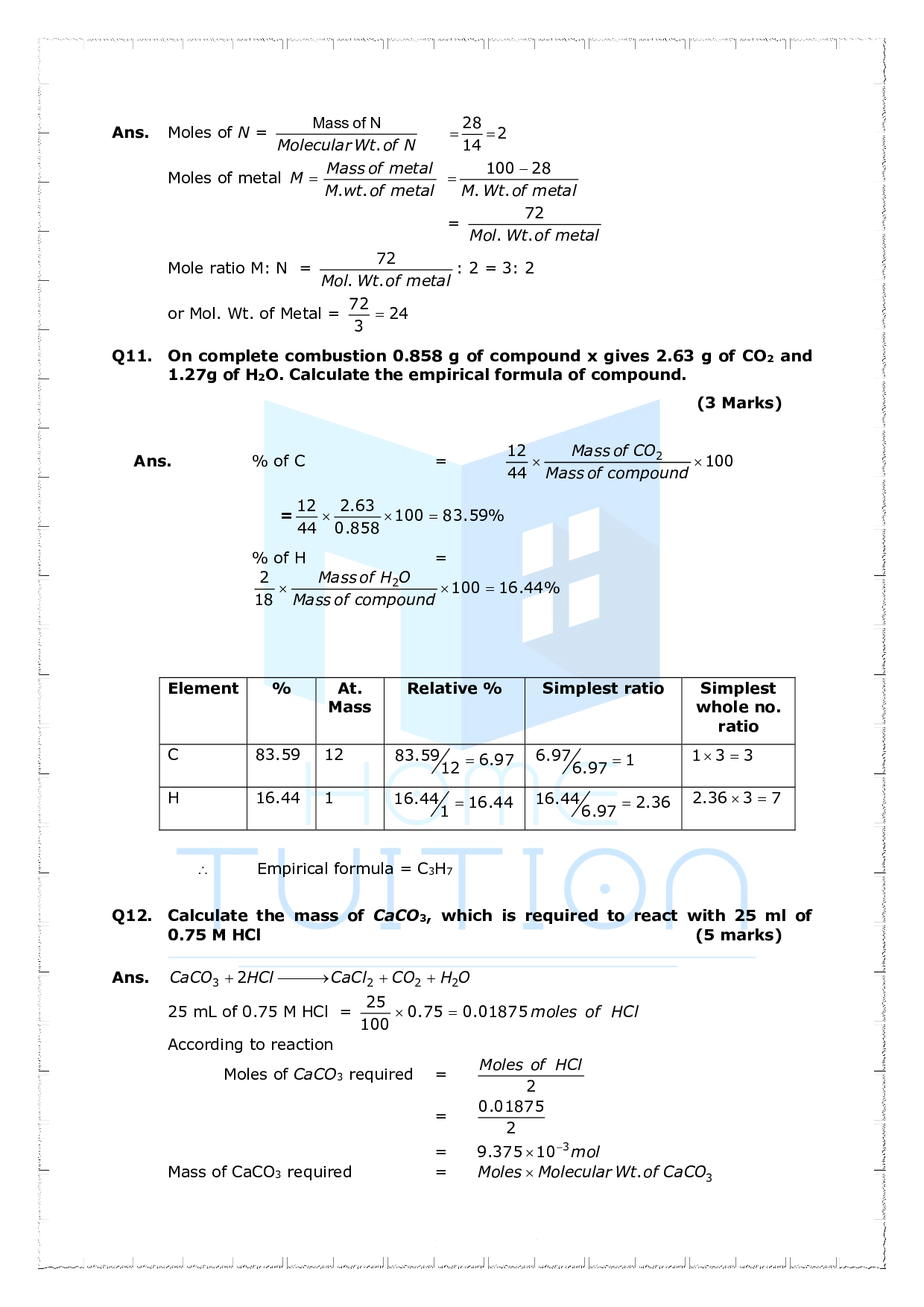
Important Questions For Class 11 Chemistry Chapter 1 Some Basic
Important Questions for CBSE Class 11 Chemistry Chapter 3 - Classification of Elements and Periodicity in Properties Last updated date: 21st Dec 2023 • Total views: 627.6k • Views today: 15.27k Download PDF NCERT Solutions CBSE CBSE Study Material Textbook Solutions CBSE Notes

39 chemical formulas and equations worksheet answers Worksheet Works
Question 1. The set representing the correct order of first ionization potential is (a) K > Na > Li (b) Be > Mg > Ca (c) B > C > N (d) Ge > Si > C. Answer Question 2. The increasing order of the a tomifc radius for the elements Na, Rb, K and Mg is (a) Na < K < Mg < Rb (b) K < Na < Mg < Rb (c) Na < Mg < K < Rb (d) Mg < Na < K < Rb Answer Question 3.

CBSE Class 11 Chemistry Chapter 3 Classification Of Elements And
Q1. Arrange s, p and d sub-shells of a shell in the increasing order of effective nuclear charge (Z eff) experienced by the electron present in them. Answer.

NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of
Chapter 3 - Classification of Elements and Periodicity in Properties Chapter 4 - Chemical Bonding and Molecular Structure Chapter 5 - States of Matter Chapter 6 - Thermodynamics Chapter 7 - Equilibrium Chapter 8 - Redox Reactions Chapter 9 - Hydrogen Chapter 10 - The s-Block Elements

Chemistry Class 11 Chapter 3 तत्त्वों का वर्गीकरण एवं गुणधर्मों में
Question 2. Which important property did Mendeleev use to classify the elements in this periodic table and did he stick to that? Answer: Mendeleev used atomic weight as the basis of classification of elements in the periodic table. He did stick to it and classify elements into groups and periods. More Resources for CBSE Class 11 NCERT Solutions

Important Questions for Class 11 Chemistry Chapter 3 Classification of
The questions covered in Chemistry Class 11 Chapter 3 important questions are based on the vital topics included this Chapter 3: Why Do We Need To Classify Elements? Genesis Of Periodic Classification Modern Periodic Law And The Present Form Of The Periodic Table Nomenclature Of Elements With Atomic Numbers >100

Class 11 Chemistry Multiple Choice Questions Part 1
With the basic understanding and regular practice, students can score a good percentage in their board exams. Some of the benefits of NCERT Solutions for Class 11 Chemistry Chapter 3 includes: Class 11 Chemistry Chapter 3 solutions are provided by teachers who have several years of experience and are professional experts in this field.

NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification
11th Chemistry - Important Unit wise 2 Mark and 3 Mark Questions (Unit 1 to 7) Mr. F. Raja - English Medium - Preview & Download (MAT.NO. 211122) 11th Chemistry - Volume 1 Important 2 Mark and 3 Mark Questions Mr. A. Dinesh - English Medium - Preview & Download (MAT.NO. 212703)

Important Questions for Class 11 Chemistry Chapter 3 Classification of
Directions : Each of these questions contain two statements, Assertion and Reason. Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.(a) Assertion is correct, reason is correct; reason is a correct explanation for. Continue reading Assertion and Reason Questions for Class.